Introduction
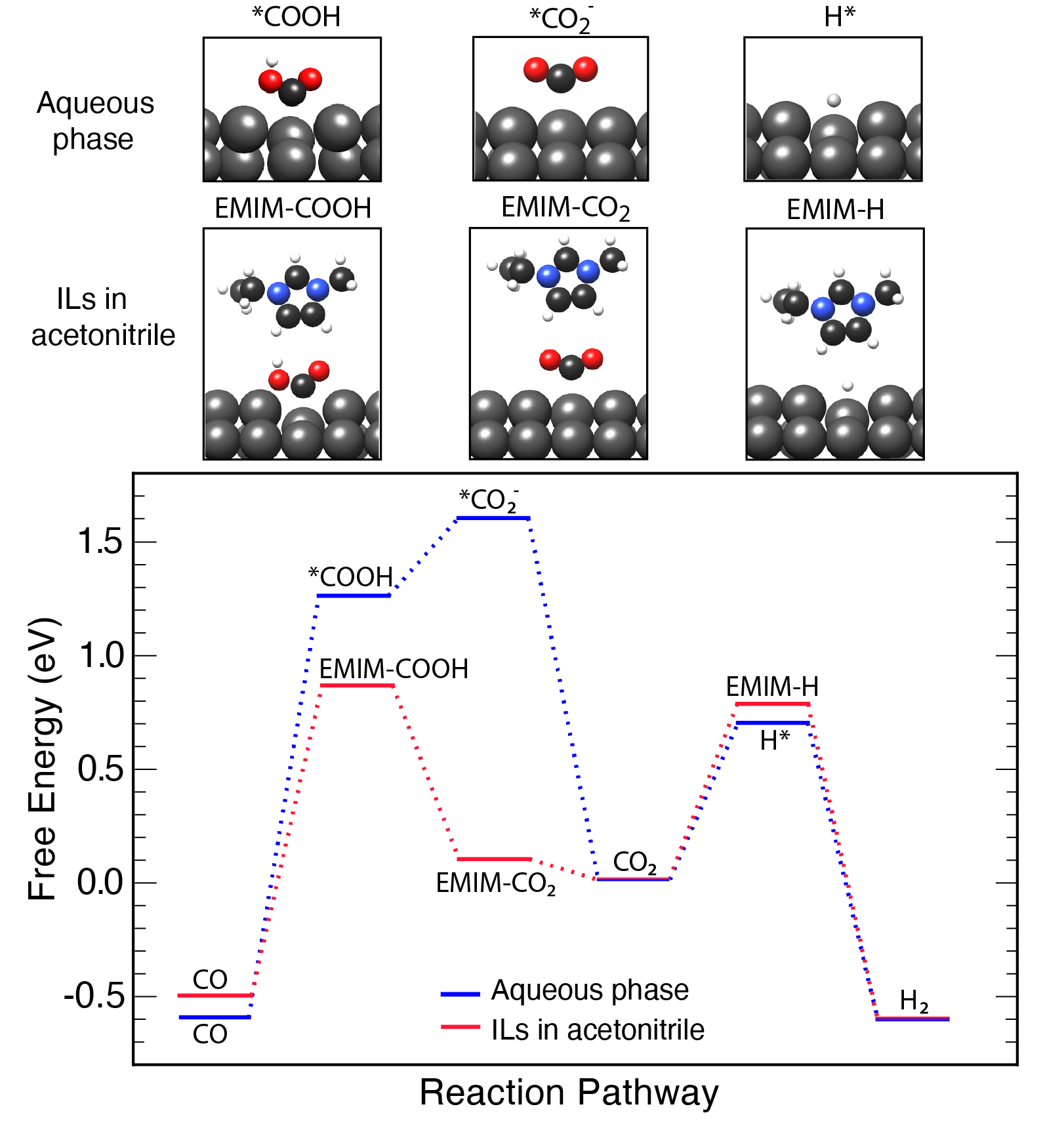
Electrochemical reduction of CO2 has attracted researchers’ attention as it has the potential to utilize the abundant greenhouse gas in the Earth’s atmosphere and store intermittent energy from solar panels and wind turbines in chemical bonds. Many metals show activities in reducing CO2 in aqueous phase. However, with the competition of the hydrogen evolution reaction, the selectivity is poor towards valued chemicals. Also, the high overpotential needed to drive the reactions causes low efficiencies that inhibits the practical applications.
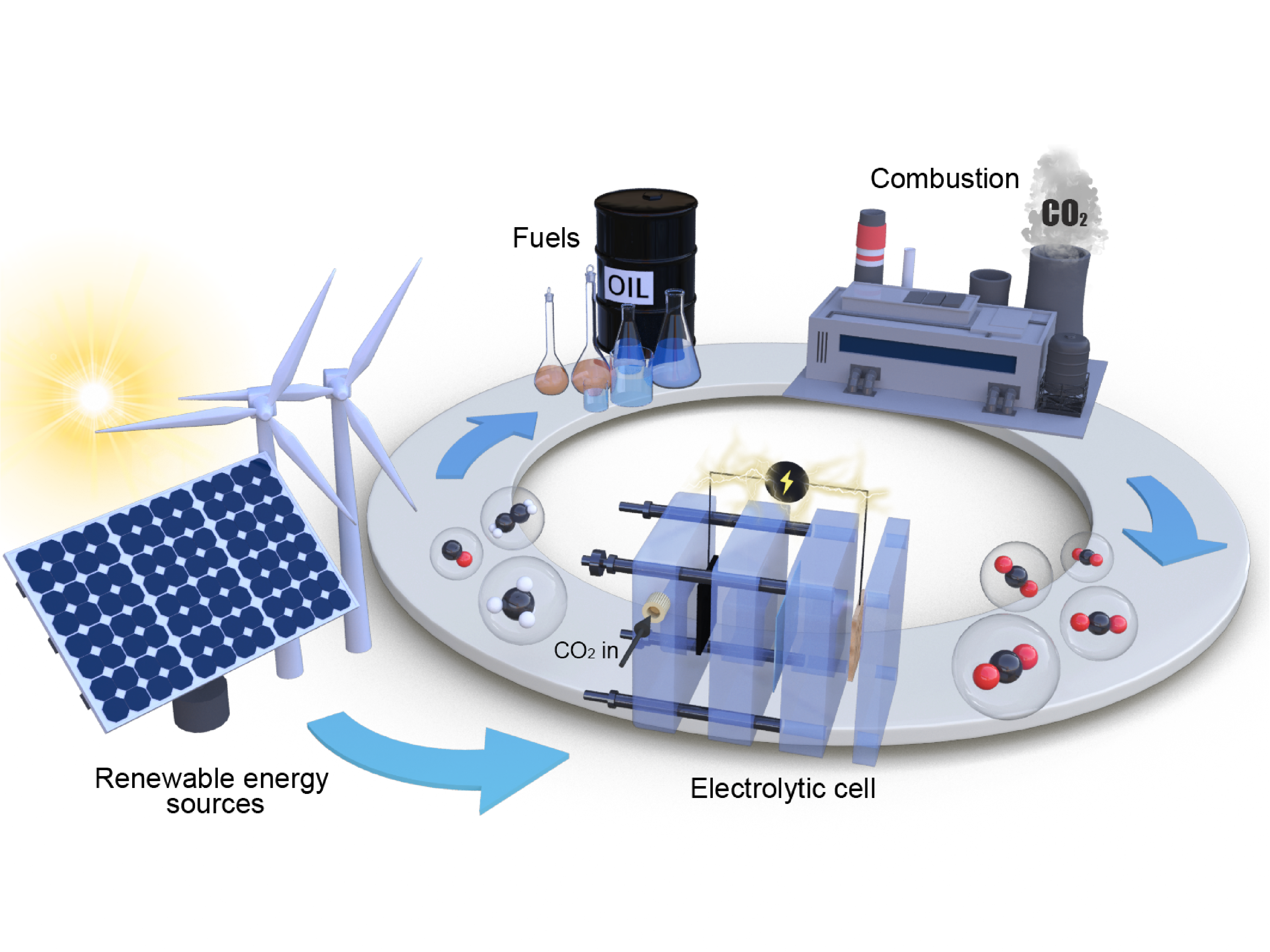
CO2 cycle
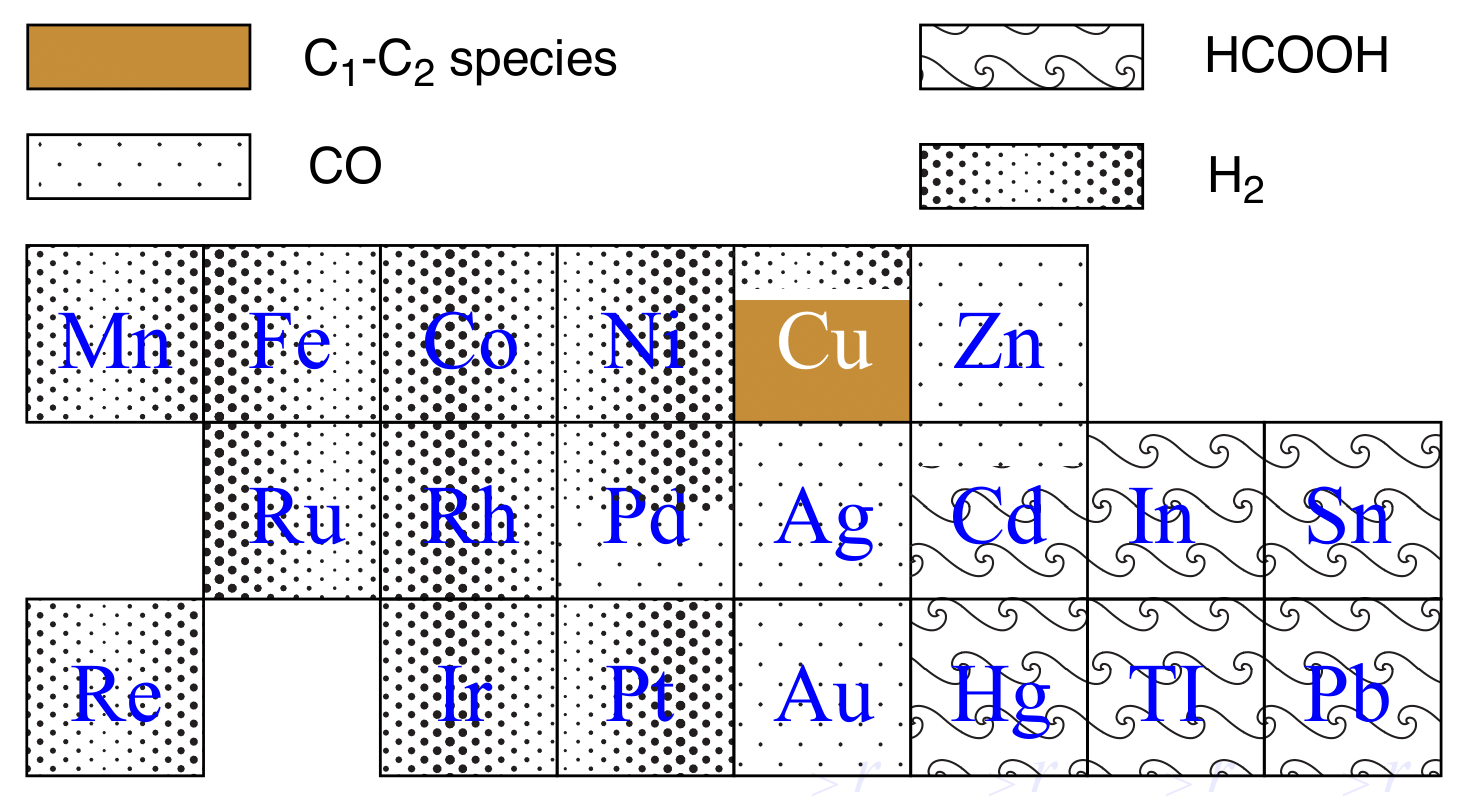
Many metals show CO22 reduction activity
Current Knowledge and Focus
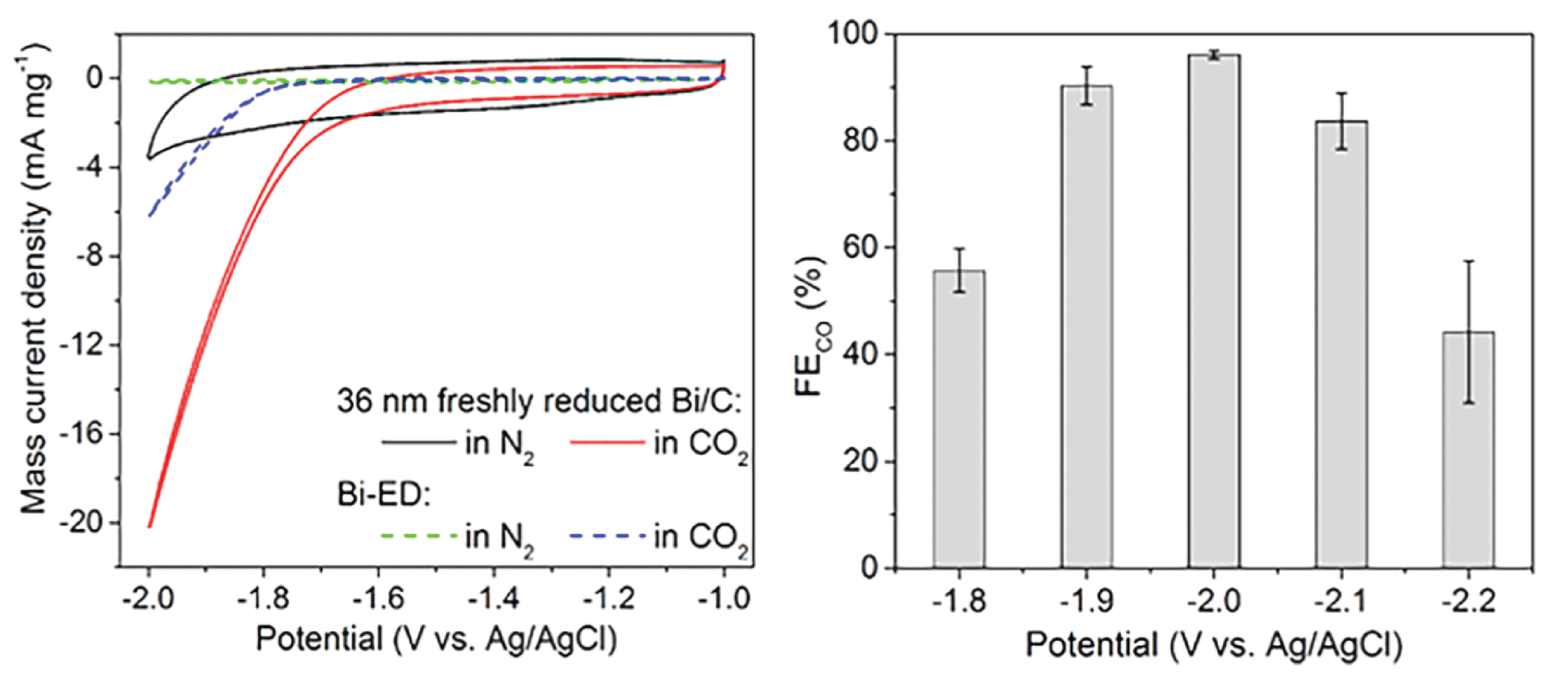
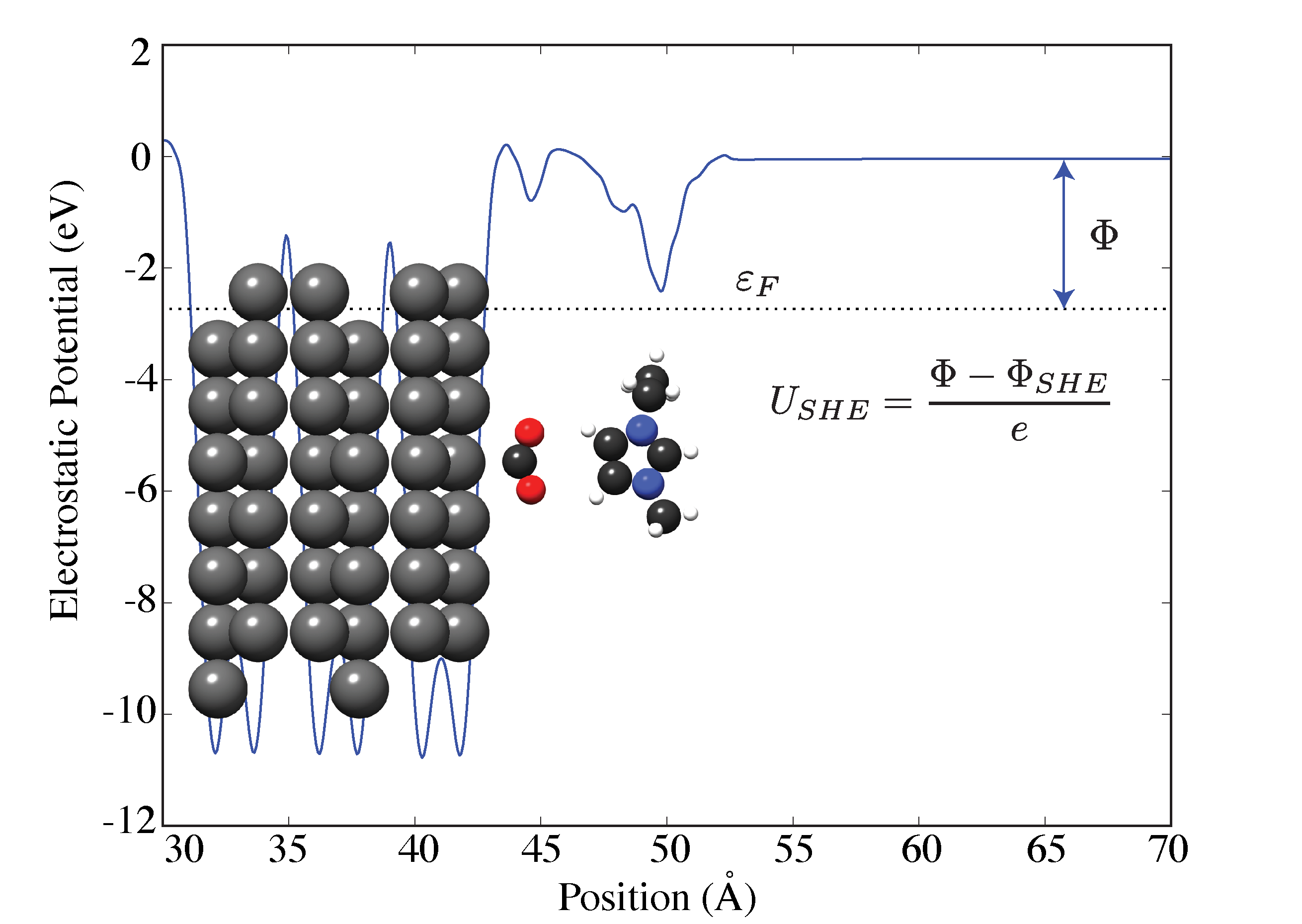
By using Bismuth... read more

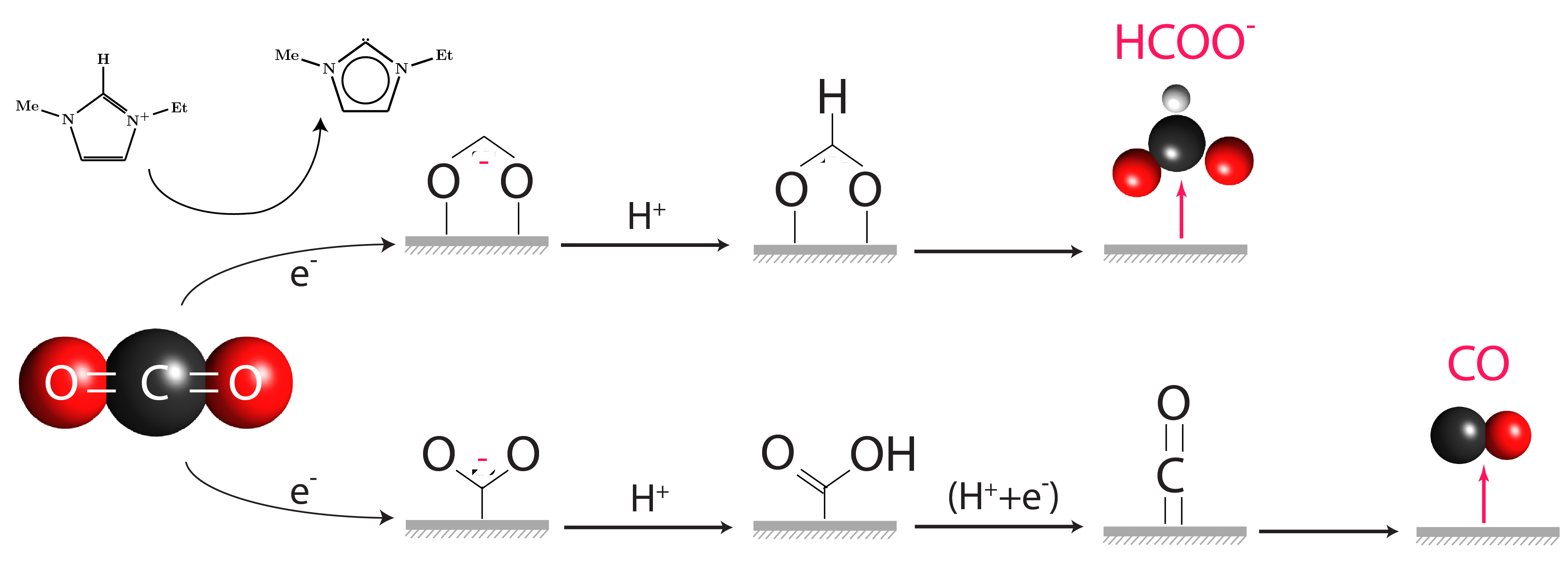
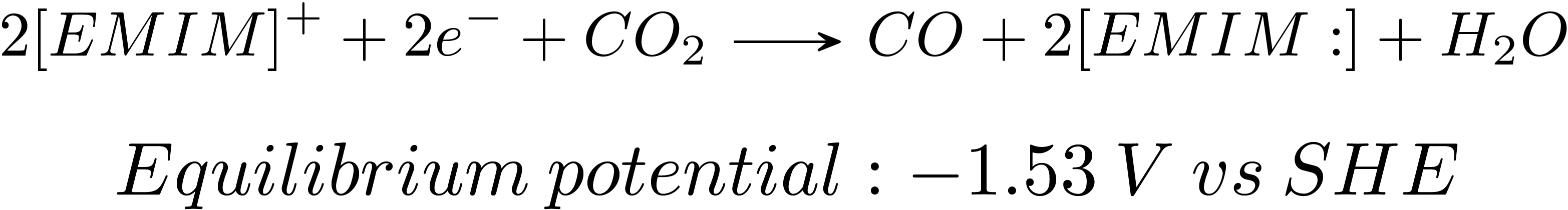
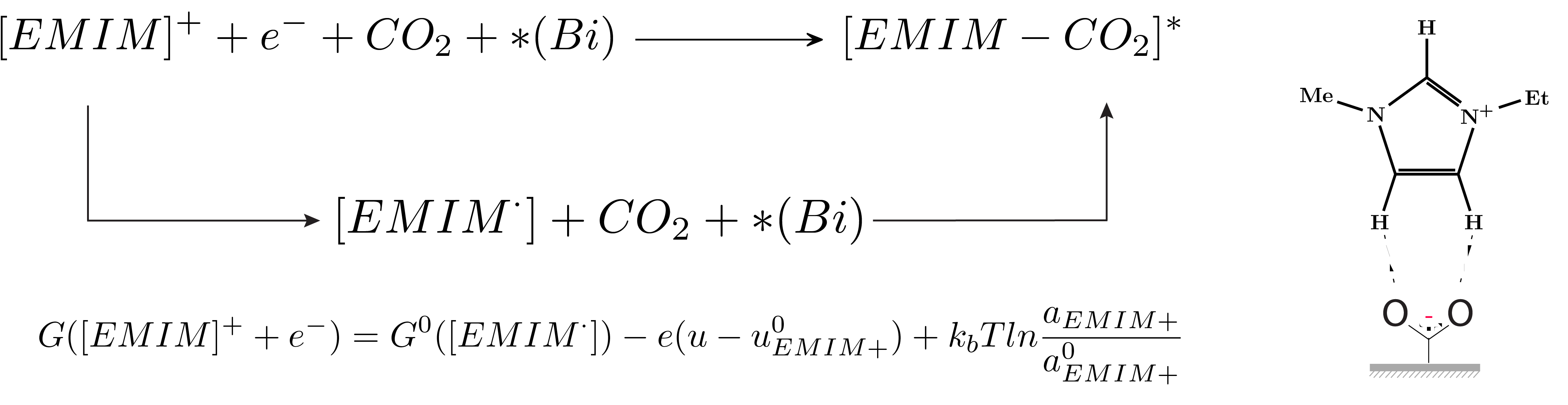

Comments